The U.S. Food and Drug Administration (FDA) has authorized a single booster dose of the Pfizer-BioNTech COVID-19 Vaccine, Bivalent for children 6 months through 4 years of age.
The FDA said in its announcement that the Pfizer-BioNTech COVID-19 Vaccine, Bivalent includes an mRNA component that provides a"broadly protective" immune response against COVID-19, as well as against the omicron variants BA.4 and BA.5.
COVID-19 SHOCKER: PARENTS LIED ABOUT THEIR KIDS’ SICKNESS STATUS AND BROKE QUARANTINE RULES, STUDY FINDS The most common side effect among children between six months and 23 months were drowsiness, irritability, pain and swelling, injection site redness, decreased appetite, fatigue and fever, the FDA noted.
South Africa Latest News, South Africa Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
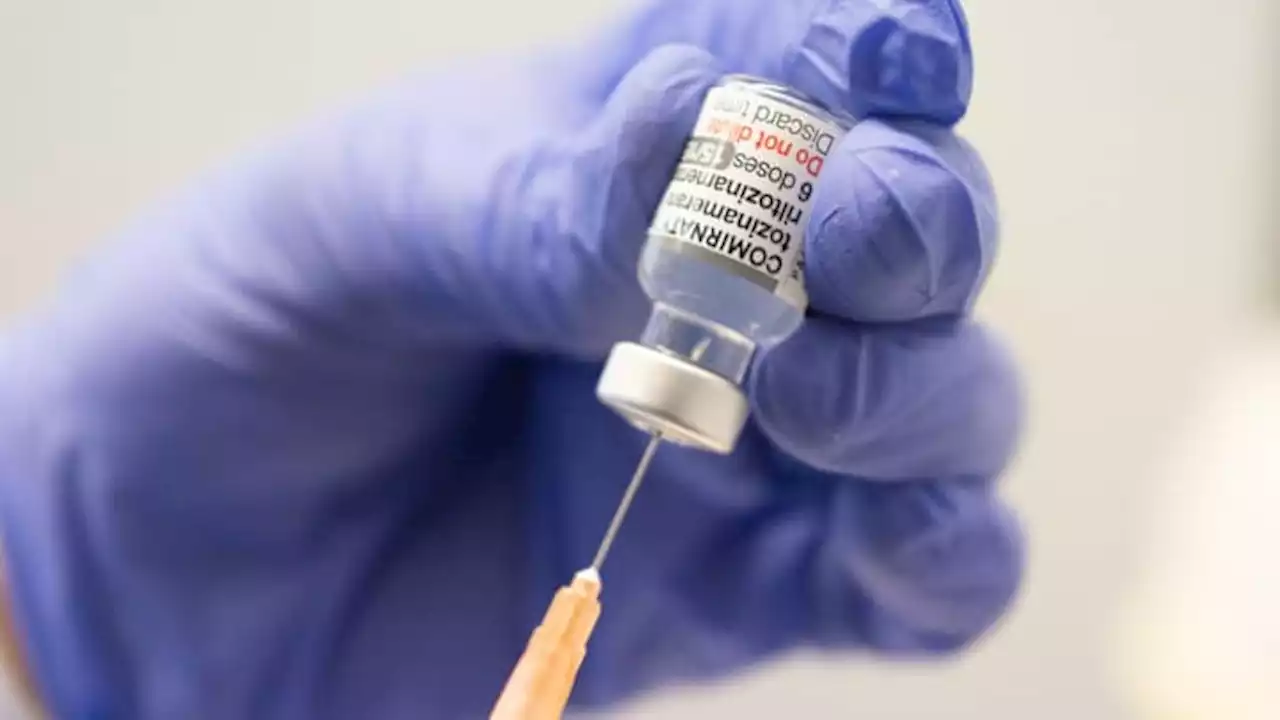 FDA authorizes Pfizer's Covid omicron booster as fourth shot for kids under 5The FDA authorized Pfizer's omicron booster shot for kids younger than five who were previously vaccinated with three doses of the company's original shot.
FDA authorizes Pfizer's Covid omicron booster as fourth shot for kids under 5The FDA authorized Pfizer's omicron booster shot for kids younger than five who were previously vaccinated with three doses of the company's original shot.
Read more »
 Pfizer receives FDA approval for migraine nasal spray, claims pain relief in 15 minutesThe drug, Zavzpret, also known as zavegepant, was approved on Thursday for the treatment of acute migraines with or without an aura in adults.
Pfizer receives FDA approval for migraine nasal spray, claims pain relief in 15 minutesThe drug, Zavzpret, also known as zavegepant, was approved on Thursday for the treatment of acute migraines with or without an aura in adults.
Read more »
 FDA updates authorization of BioNTech, Pfizer’s bivalent booster for young childrenThe Food and Drug Administration on Tuesday said children between the ages of 6 months and 5 years old can now get a booster dose of BioNTech and Pfizer’s...
FDA updates authorization of BioNTech, Pfizer’s bivalent booster for young childrenThe Food and Drug Administration on Tuesday said children between the ages of 6 months and 5 years old can now get a booster dose of BioNTech and Pfizer’s...
Read more »
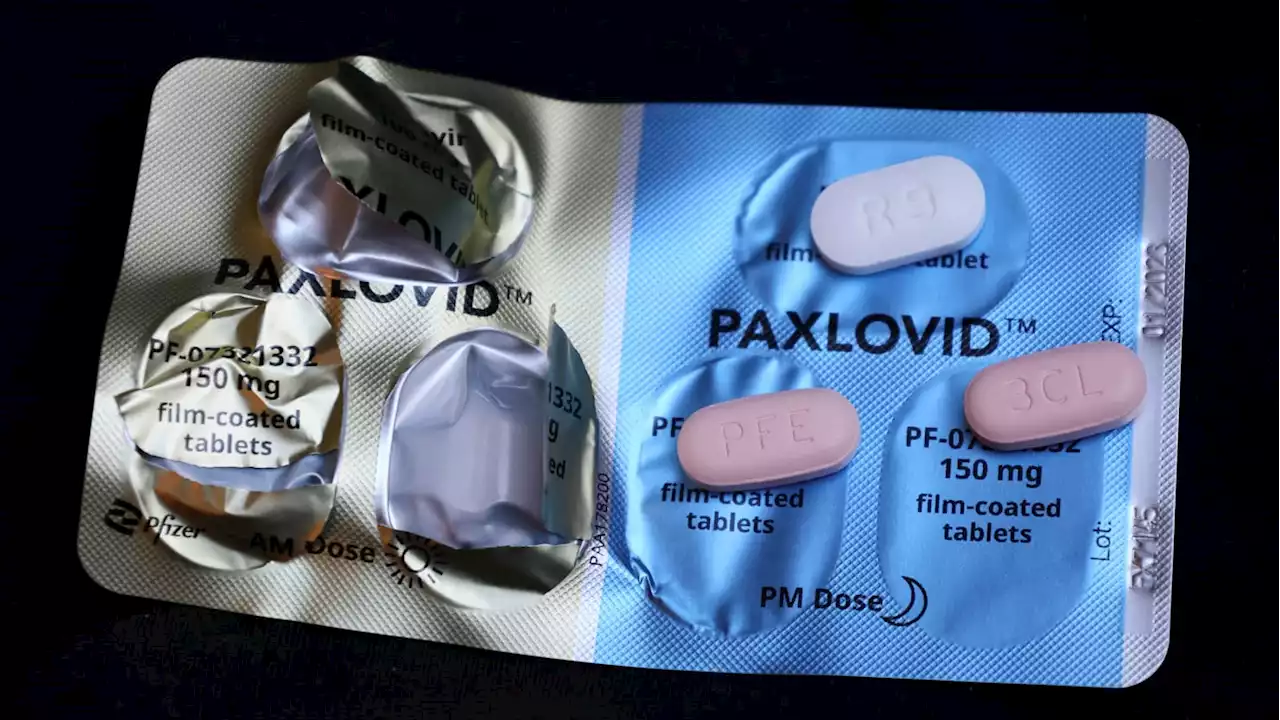 FDA: Paxlovid Does Not Cause COVID ‘Rebound’The FDA says in a new report that rebound also occurs in people who did not take paxlovid—which can prevent COVID from becoming severe if given early enough—and appears to be part of the disease process in some.
FDA: Paxlovid Does Not Cause COVID ‘Rebound’The FDA says in a new report that rebound also occurs in people who did not take paxlovid—which can prevent COVID from becoming severe if given early enough—and appears to be part of the disease process in some.
Read more »
 FDA: Paxlovid not associated with COVID reboundPaxlovid isn't associated with COVID rebound, in which patients test positive or have symptoms days after a course of the drug is completed, Food and Drug Administration staff said Tuesday.
FDA: Paxlovid not associated with COVID reboundPaxlovid isn't associated with COVID rebound, in which patients test positive or have symptoms days after a course of the drug is completed, Food and Drug Administration staff said Tuesday.
Read more »
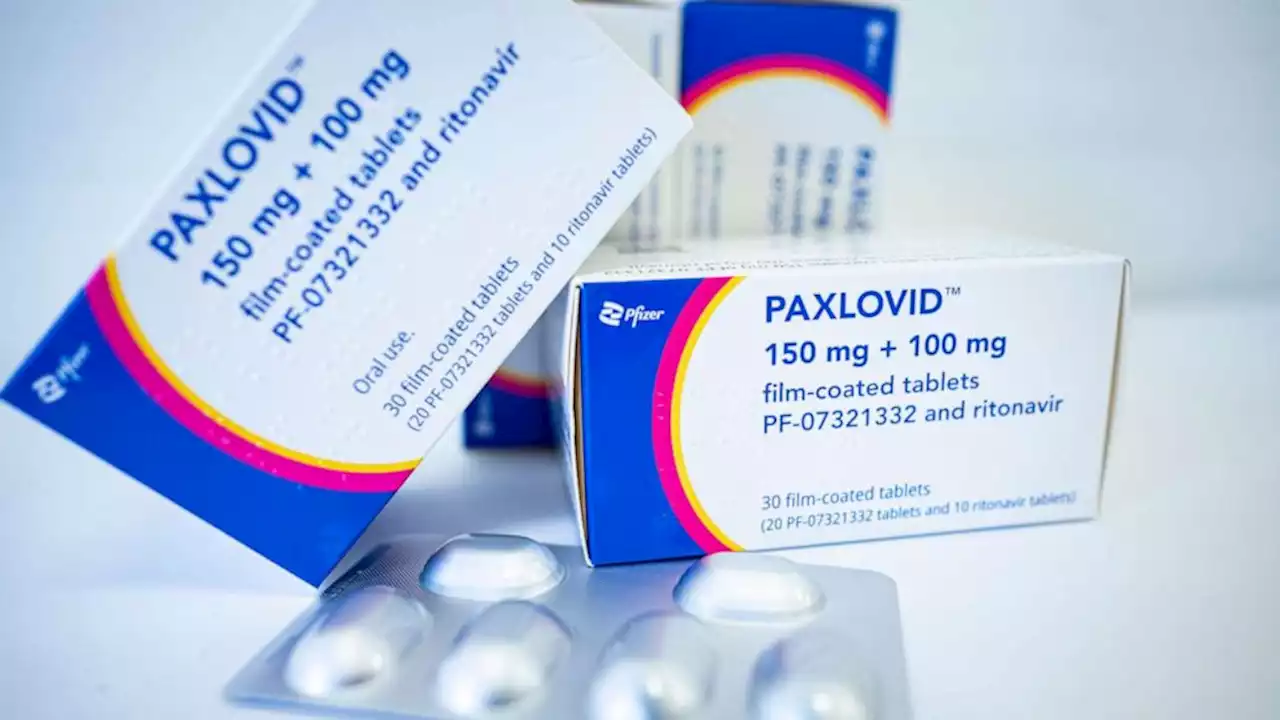 No clear association between Paxlovid and COVID-19 rebound, FDA saysAn FDA analysis did not find a clear association between the COVID-19 antiviral drug Paxlovid and illness rebound, the FDA said in a new report.
No clear association between Paxlovid and COVID-19 rebound, FDA saysAn FDA analysis did not find a clear association between the COVID-19 antiviral drug Paxlovid and illness rebound, the FDA said in a new report.
Read more »
