The FDA says in a new report that rebound also occurs in people who did not take paxlovid—which can prevent COVID from becoming severe if given early enough—and appears to be part of the disease process in some.
Pfizer’s antiviral drug used to treat COVID in higher-risk patients has been dogged by complaints that it causes a rebound effect in which people get symptoms again.
But the FDA says in a new report that rebound also occurs in people who did not take—which can prevent COVID from becoming severe if given early enough—and appears to be part of the disease process in some. The FDA reviewed Pfizer’s clinical trials of paxlovid ahead of an an advisory committee meeting on whether to recommend full approval of the drug.
South Africa Latest News, South Africa Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 “COVID Rebound” Is Common – Even in Patients Not Treated With PaxlovidPreliminary results from a Scripps Research and eMed digital medicine study show an unexpectedly high proportion of COVID-19 rebound cases in untreated people, as well as those treated with Paxlovid. “COVID rebound,” in which evidence of the illness disappears and then returns days or weeks later
“COVID Rebound” Is Common – Even in Patients Not Treated With PaxlovidPreliminary results from a Scripps Research and eMed digital medicine study show an unexpectedly high proportion of COVID-19 rebound cases in untreated people, as well as those treated with Paxlovid. “COVID rebound,” in which evidence of the illness disappears and then returns days or weeks later
Read more »
 The FDA Just Approved The First Fast-Acting Nasal Spray For MigrainesThe US Food and Drug Administration has approved a fast-acting nasal spray from Pfizer designed to treat migraines, the US pharmaceutical giant said Friday.
The FDA Just Approved The First Fast-Acting Nasal Spray For MigrainesThe US Food and Drug Administration has approved a fast-acting nasal spray from Pfizer designed to treat migraines, the US pharmaceutical giant said Friday.
Read more »
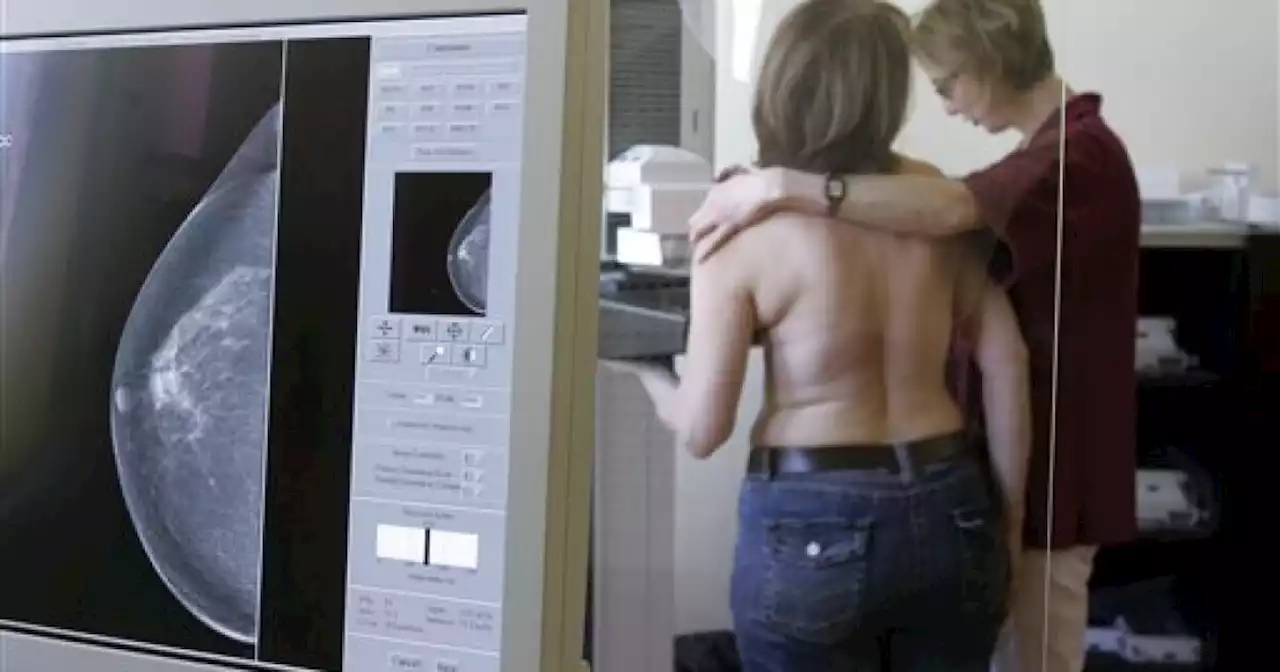 New FDA standards improve health, help strengthen the fight against breast cancerIn the next 18 months, the FDA will require mammogram facilities to let patients know if they have dense breast tissue so they can consult with a doctor if they need additional screening.
New FDA standards improve health, help strengthen the fight against breast cancerIn the next 18 months, the FDA will require mammogram facilities to let patients know if they have dense breast tissue so they can consult with a doctor if they need additional screening.
Read more »
 FDA Approves Fast-Acting Nasal Spray for Migraines, Pfizer SaysClinical trials showed the nasal spray provided migraine relief within 15-30 minutes of use, with the relief lasting up to 48 hours in many patients.
FDA Approves Fast-Acting Nasal Spray for Migraines, Pfizer SaysClinical trials showed the nasal spray provided migraine relief within 15-30 minutes of use, with the relief lasting up to 48 hours in many patients.
Read more »
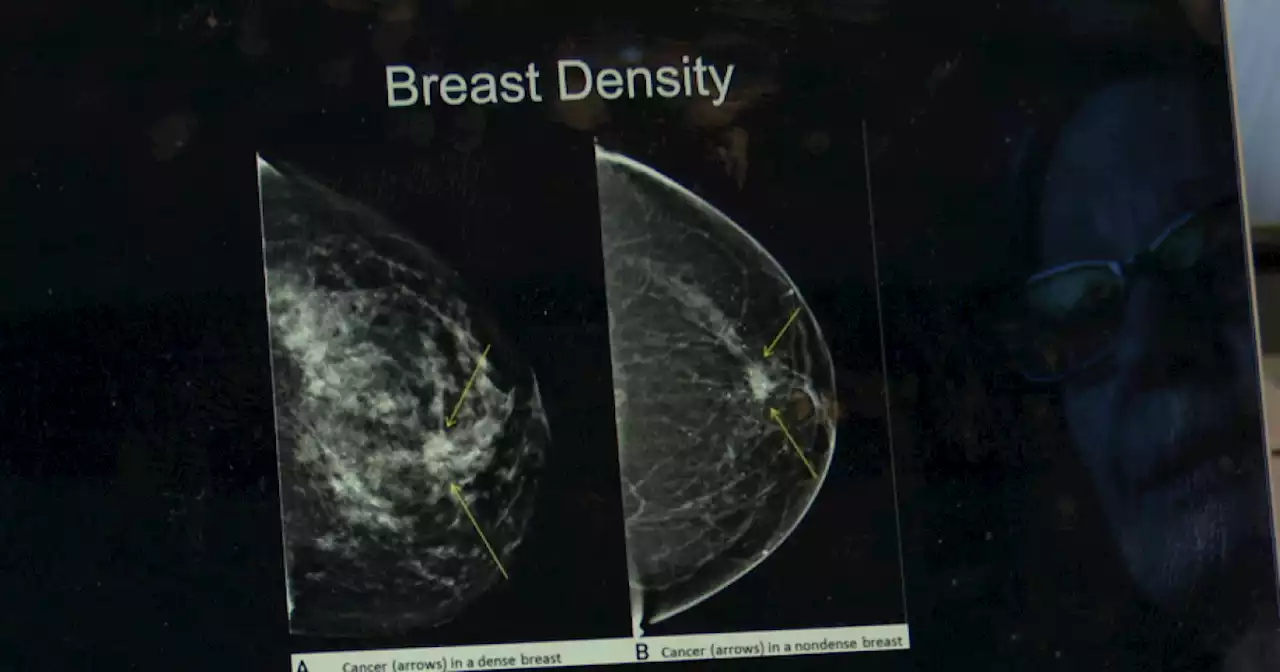 FDA updates mammogram guidelines in hopes to save livesDr. Foster recommends getting a mammogram once a year after you turn 40. About one in eight women will develop breast cancer in their life according to the CDC.
FDA updates mammogram guidelines in hopes to save livesDr. Foster recommends getting a mammogram once a year after you turn 40. About one in eight women will develop breast cancer in their life according to the CDC.
Read more »
 First FDA-cleared automated ear cleaner could become widely usedAn FDA-cleared automated ear cleaning system looks like a pair of headphones, and could become a widely used part of more automated healthcare.
First FDA-cleared automated ear cleaner could become widely usedAn FDA-cleared automated ear cleaning system looks like a pair of headphones, and could become a widely used part of more automated healthcare.
Read more »
