A panel of U.S. Food and Drug Administration advisers on Friday voted for the restricted use of British drugmaker AztraZeneca Plc's experimental treatment, jointly developed with Merck & Co , for a type of prostate cancer.
They were withdrawn last year as second line of treatment for ovarian cancer patients after the FDA limited the use of PARP inhibitors and requested companies to pull the drug.
Friday's vote is based on a late-stage study, which showed Lynparza in combination significantly improved duration for which patients live without disease worsening when compared to the placebo in combination with abiraterone and prednisone/prednisolone. Lynparza is already approved by the FDA to treat a type of breast cancer, ovarian cancer, as well as a different form of prostate cancer.
Prostate cancer is the most common form of cancer among men in the United States with about 288,300 new cases in 2023, according to the American Cancer Society's estimates. The FDA, which usually follows the recommendations of its expert panel but is not obligated to do so, will make its final decision on the use of the drug.Our Standards:
South Africa Latest News, South Africa Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
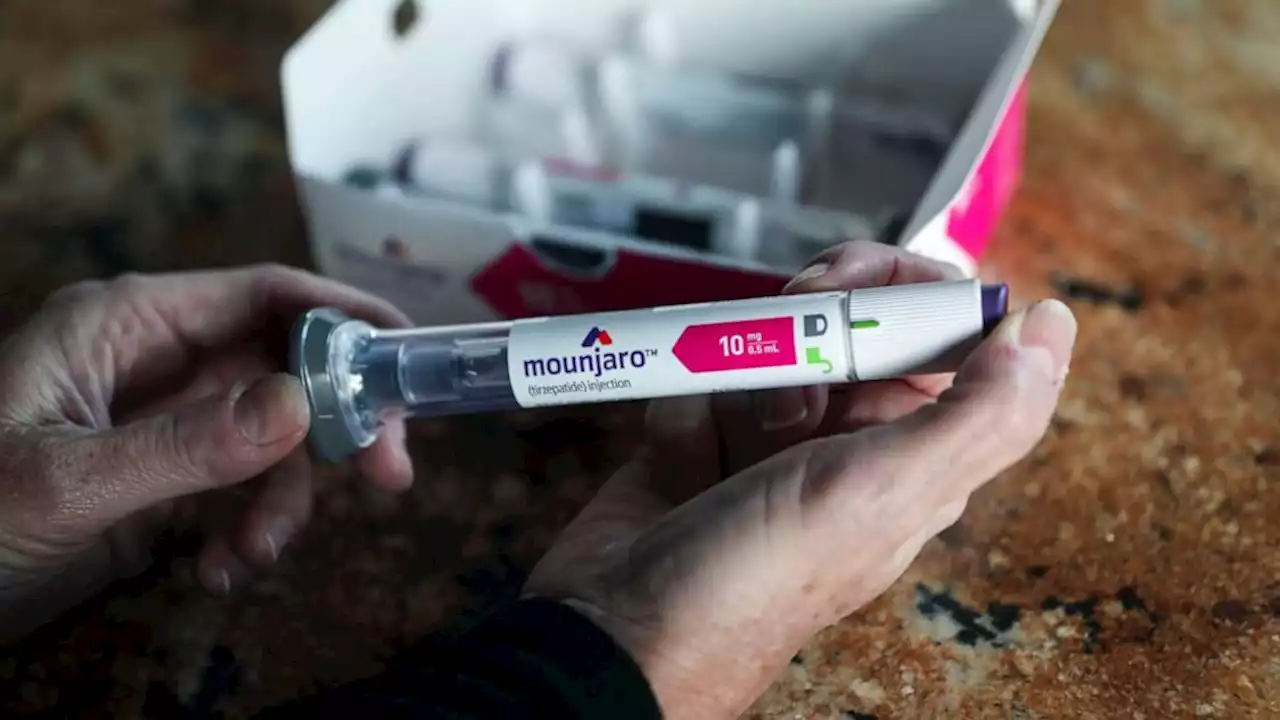 Popular diabetes drug Mounjaro could be FDA-approved for weight loss this year, company saysA popular drug currently approved to treat Type 2 diabetes could soon also be approved for weight loss.
Popular diabetes drug Mounjaro could be FDA-approved for weight loss this year, company saysA popular drug currently approved to treat Type 2 diabetes could soon also be approved for weight loss.
Read more »
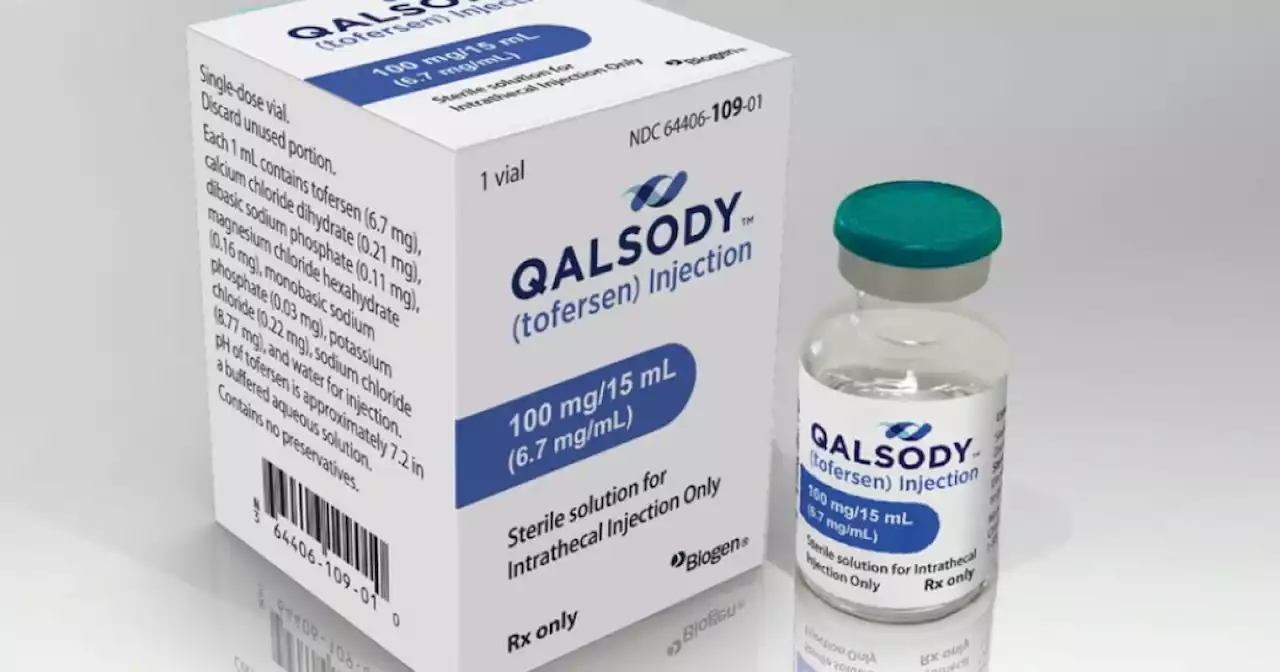 FDA approves Biogen's drug for rare form of ALSThe U.S. Food and Drug Administration has approved a new drug to treat a rare form of Amyotrophic Lateral Sclerosis (ALS), also commonly known as Lou Gehrig’s disease.
FDA approves Biogen's drug for rare form of ALSThe U.S. Food and Drug Administration has approved a new drug to treat a rare form of Amyotrophic Lateral Sclerosis (ALS), also commonly known as Lou Gehrig’s disease.
Read more »
 AstraZeneca says new COVID drug could guard against all variants of concern to dateThe drugmaker says the FDA could clear the new COVID drug for immunocompromised in months.
AstraZeneca says new COVID drug could guard against all variants of concern to dateThe drugmaker says the FDA could clear the new COVID drug for immunocompromised in months.
Read more »
 New report shows Travis County fentanyl deaths have doubled since 2021The number of cases reported as fentanyl-related deaths in 2022 was 245, whereas in 2021 there were 118. In years prior, cases weren’t even near the triple digits.
New report shows Travis County fentanyl deaths have doubled since 2021The number of cases reported as fentanyl-related deaths in 2022 was 245, whereas in 2021 there were 118. In years prior, cases weren’t even near the triple digits.
Read more »
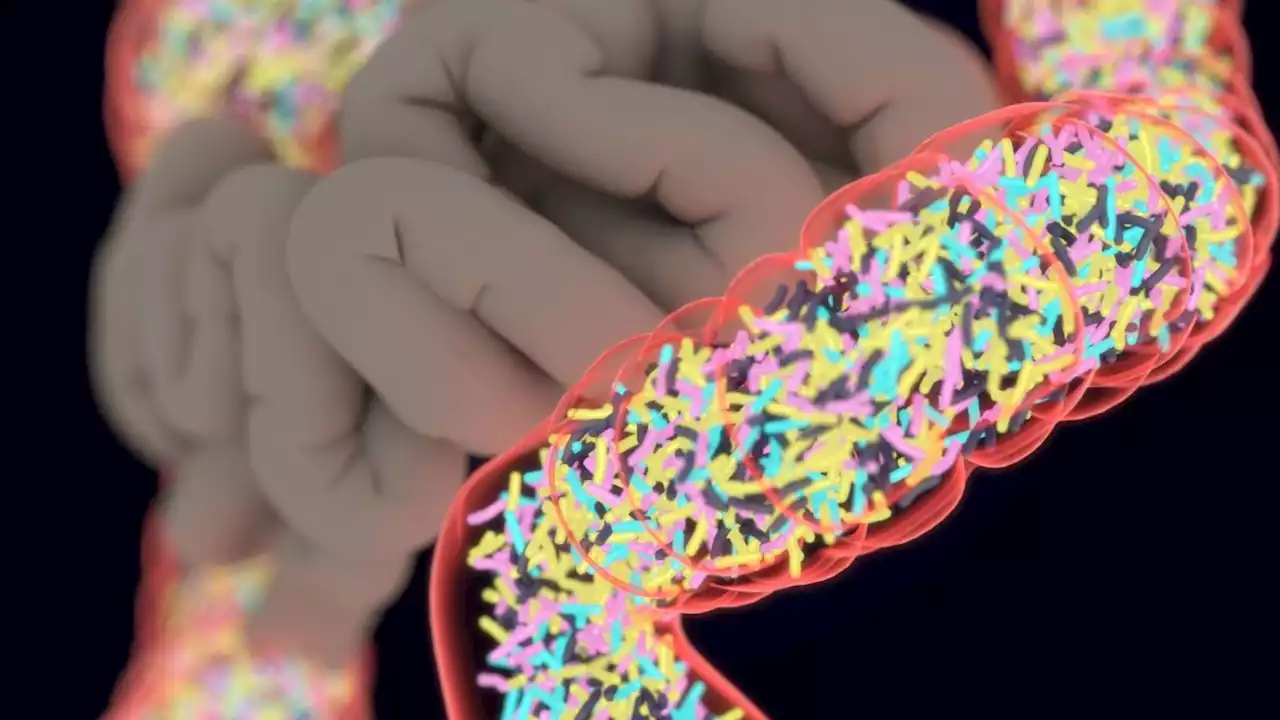 FDA approves 1st pill made from human poopThe Food and Drug Administration approved the second-ever medical treatment made from donated human feces.
FDA approves 1st pill made from human poopThe Food and Drug Administration approved the second-ever medical treatment made from donated human feces.
Read more »
 US FDA approves Seres Therapeutics' pill for deadly C. difficile infectionsThe U.S. health regulator on Wednesday approved Seres Therapeutics Inc's pill for treating a type of bacterial infection, giving an easier and standardized option to patients who often have to rely on individual donors for fecal transplants.
US FDA approves Seres Therapeutics' pill for deadly C. difficile infectionsThe U.S. health regulator on Wednesday approved Seres Therapeutics Inc's pill for treating a type of bacterial infection, giving an easier and standardized option to patients who often have to rely on individual donors for fecal transplants.
Read more »
