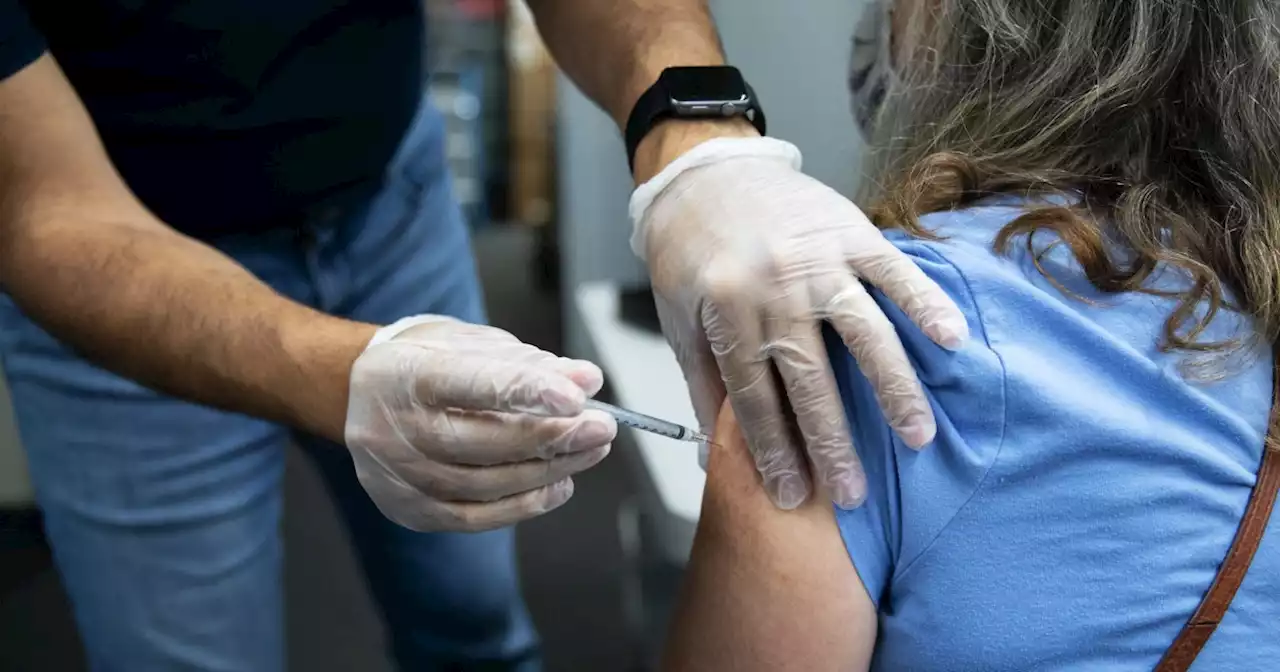
Federal health officials are continuing to monitor whether another booster dose will be needed and, if so, when.
Many health experts agree additional doses will be needed in the future, though how often remains up for debate, said Dr. Archana Chatterjee, a vaccine expert and dean of the Chicago Medical School at Rosalind Franklin University.
And if the additional shots are needed, they likely won't be recommended for everyone, said Dr. Paul Offit, a vaccine expert at Children’s Hospital of Philadelphia. Bill Hanage, a Harvard epidemiologist, agreed that an additional dose may be needed for certain groups, saying antibody levels may wane further over the summer, when the virus could continue to spread at low levels.
South Africa Latest News, South Africa Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Phase 2, 3 trial for Pfizer pill for COVID begins in children ages 6 to 17'There is a significant unmet need for outpatient treatments that can be taken by children and adolescents to help prevent progression to severe illness, including hospitalization or death.'
Phase 2, 3 trial for Pfizer pill for COVID begins in children ages 6 to 17'There is a significant unmet need for outpatient treatments that can be taken by children and adolescents to help prevent progression to severe illness, including hospitalization or death.'
Read more »
 Pfizer launches COVID pill clinical trial for childrenPfizer announced Wednesday that it has begun clinical trials of its COVID-19 pills in children ages 6 to 17.
Pfizer launches COVID pill clinical trial for childrenPfizer announced Wednesday that it has begun clinical trials of its COVID-19 pills in children ages 6 to 17.
Read more »
 Pfizer begins next phases of trial of antiviral COVID-19 pill for childrenPfizer announced Wednesday it has begun phases 2 and 3 of its pediatric clinical trial for Paxlovid, its COVID-19 antiviral treatment.
Pfizer begins next phases of trial of antiviral COVID-19 pill for childrenPfizer announced Wednesday it has begun phases 2 and 3 of its pediatric clinical trial for Paxlovid, its COVID-19 antiviral treatment.
Read more »
 Pfizer Starts Next Phases Of COVID-19 Antiviral Trial In KidsPfizer has begun a phase 2 and phase 3 trial of Paxlovid, a COVID-19 treatment, in children ages 6 to 17.
Pfizer Starts Next Phases Of COVID-19 Antiviral Trial In KidsPfizer has begun a phase 2 and phase 3 trial of Paxlovid, a COVID-19 treatment, in children ages 6 to 17.
Read more »
 Utah reports fewer than 200 new COVID-19 cases for 4th consecutive dayThe Utah Department of Health on Wednesday also reported nine more COVID-19 deaths for a total of 4,494 since the pandemic began.
Utah reports fewer than 200 new COVID-19 cases for 4th consecutive dayThe Utah Department of Health on Wednesday also reported nine more COVID-19 deaths for a total of 4,494 since the pandemic began.
Read more »
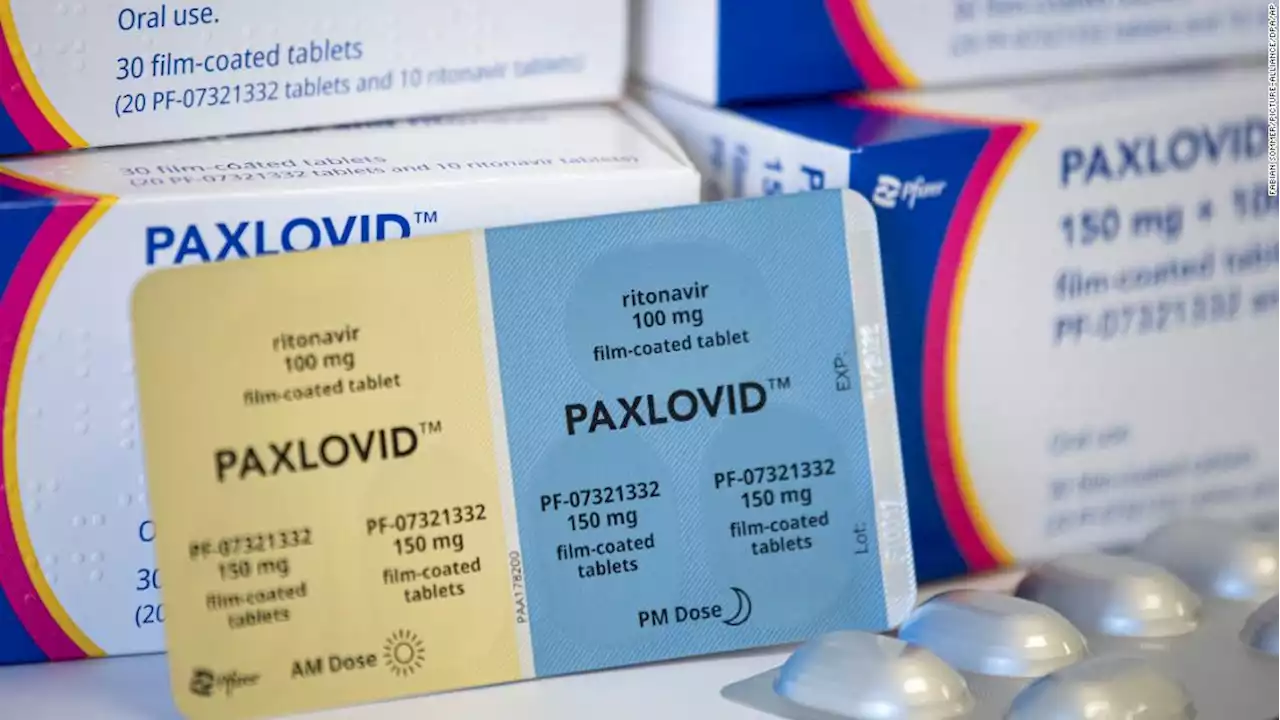 Pfizer begins Phase 2 and 3 trial of Covid-19 antiviral Paxlovid in children ages 6 to 17Pfizer has begun a Phase 2 and 3 clinical trial of its Covid-19 antiviral treatment, Paxlovid, in children ages 6 to 17, the company said Wednesday in a news release.
Pfizer begins Phase 2 and 3 trial of Covid-19 antiviral Paxlovid in children ages 6 to 17Pfizer has begun a Phase 2 and 3 clinical trial of its Covid-19 antiviral treatment, Paxlovid, in children ages 6 to 17, the company said Wednesday in a news release.
Read more »