.Pfizer-BioNTech_Group asks US_FDA to authorize omicron booster for children under 5
Children within that age group would receive the omicron boosters as the third shot in their primary series if approved, the vaccine manufacturers said.The primary series for children younger than 5 currently includes three doses of the original COVID-19 vaccine, while the primary series for those over 5 only involves two doses.
"With the high level of respiratory illnesses currently circulating among children under 5 years of age, updated COVID-19 vaccines may help prevent severe illness and hospitalization," Pfizer and BioNTech said Monday. The request comes as a surge in respiratory illnesses, including the flu and respiratory syncytial virus, or RSV, has led to overcrowding in some children's hospitals across the country.
Pfizer and BioNTech's omicron booster has already been authorized for people 5 and older in the United States and the European Union. Even if omicron boosters are authorized for the younger age group, uptake for the updated shots could be low, as only 10% of children 6 months through 4 years have received their first COVID-19 vaccine dose, according to the Centers for Disease Control and Prevention.Meanwhile, the emergence of new coronavirus subvariants BQ.1 and BQ.1.1 might affect the effectiveness of the updated boosters.
South Africa Latest News, South Africa Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Pfizer/BioNTech seek FDA authorization of updated Covid-19 vaccine for children under 5 | CNNPfizer and BioNTech have submitted an application to the US Food and Drug Administration for their updated Covid-19 vaccine to be used as the third shot in the three-dose primary vaccine series for children ages 6 months through 4 years.
Pfizer/BioNTech seek FDA authorization of updated Covid-19 vaccine for children under 5 | CNNPfizer and BioNTech have submitted an application to the US Food and Drug Administration for their updated Covid-19 vaccine to be used as the third shot in the three-dose primary vaccine series for children ages 6 months through 4 years.
Read more »
 Pfizer, BioNTech ask FDA to authorize bivalent COVID vaccine in young childrenPfizer Inc. undefined and BioNTech SE undefined said Monday that they asked the Food and Drug Administration to authorize their updated COVID-19 vaccine as...
Pfizer, BioNTech ask FDA to authorize bivalent COVID vaccine in young childrenPfizer Inc. undefined and BioNTech SE undefined said Monday that they asked the Food and Drug Administration to authorize their updated COVID-19 vaccine as...
Read more »
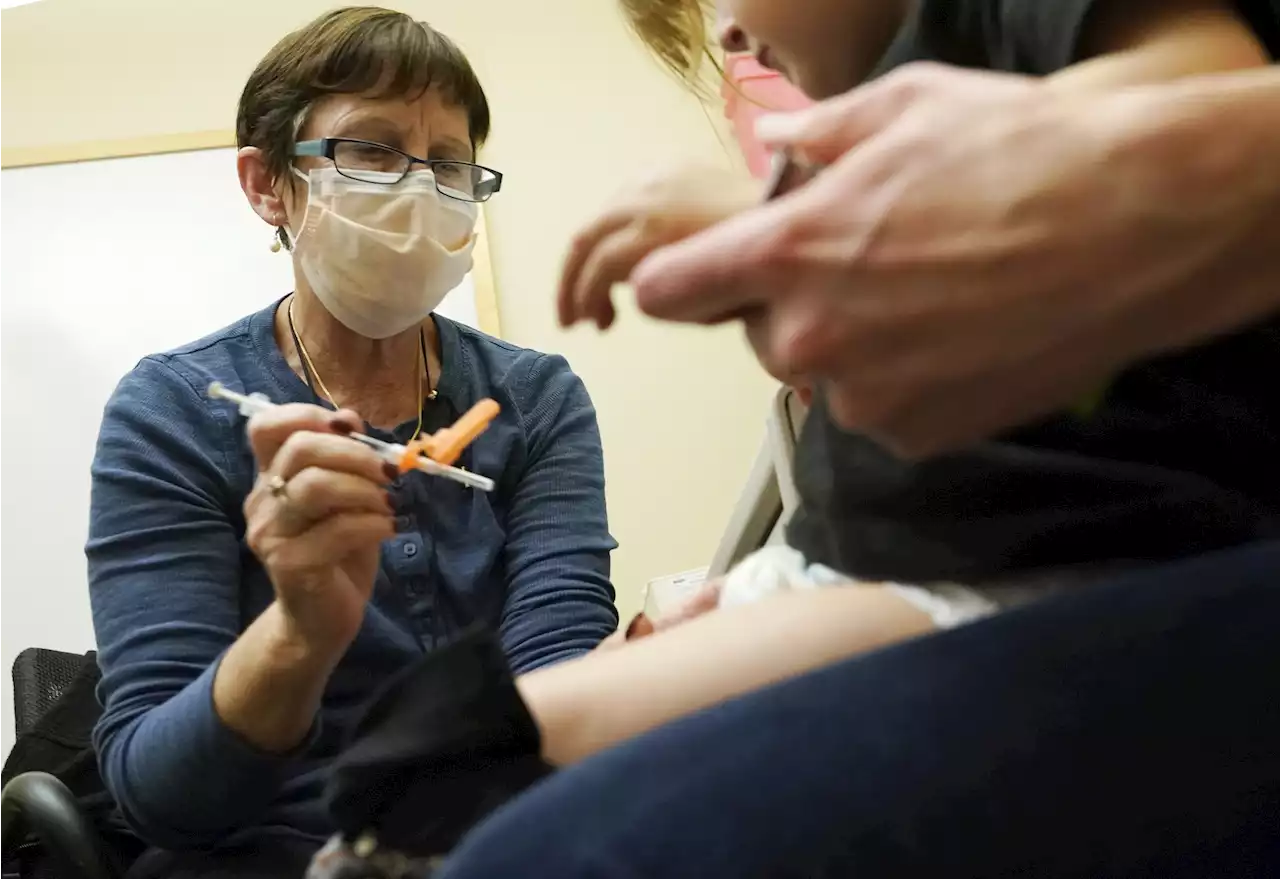 Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5 — not as a booster but part of their initial shots. Children ages 6 months through 4 years already are supposed to get three extra-small doses of the original Pfizer COVID-19 vaccine — each a tenth of the amount adults receive — as their primary series.
Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5 — not as a booster but part of their initial shots. Children ages 6 months through 4 years already are supposed to get three extra-small doses of the original Pfizer COVID-19 vaccine — each a tenth of the amount adults receive — as their primary series.
Read more »
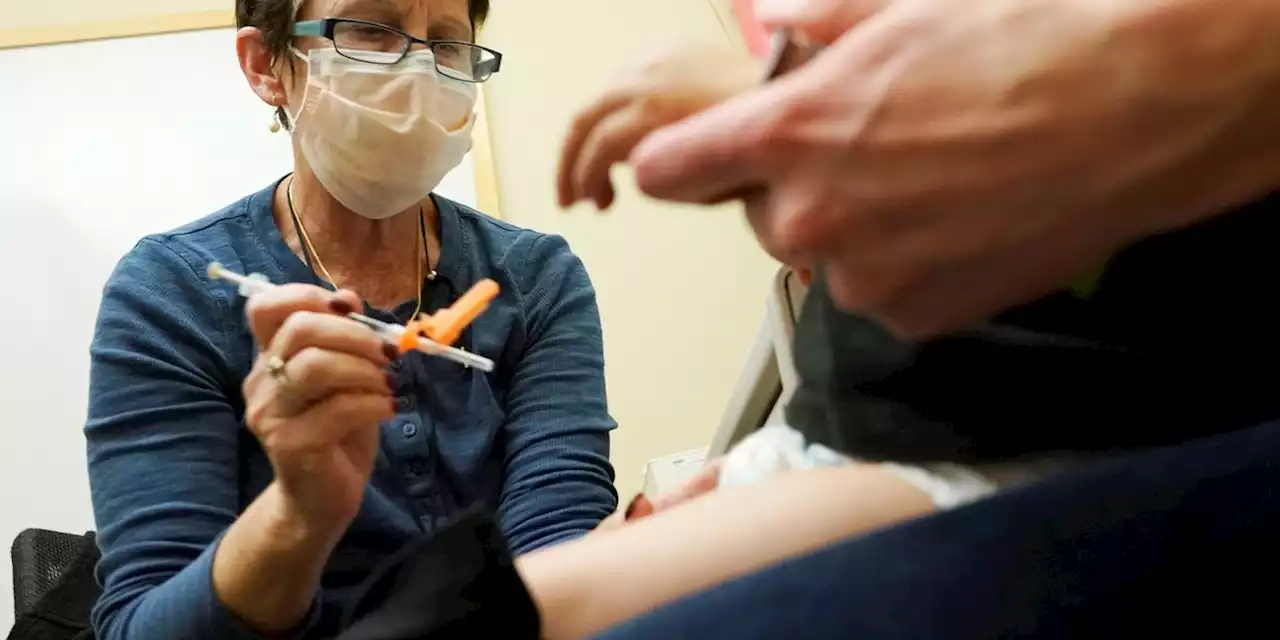 Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer and its partner BioNTech said Monday that may help prevent severe illness and hospitalization from COVID-19 in little kids, at a time when children’s hospitals already are packed with youngsters hit by other respiratory illnesses.
Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer and its partner BioNTech said Monday that may help prevent severe illness and hospitalization from COVID-19 in little kids, at a time when children’s hospitals already are packed with youngsters hit by other respiratory illnesses.
Read more »
 Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5.
Pfizer asks FDA to clear updated COVID shot for kids under 5Pfizer is asking U.S. regulators to authorize its updated COVID-19 vaccine for children under age 5.
Read more »
 Pfizer applies for FDA authorization for Omicron-retooled vaccine booster in kids under 5Pfizer Inc and its German partner BioNTech SE said on Monday they have submitted an application to the U.S. Food and Drug Administration (FDA) for emergency use authorization of their Omicron-adapted COVID-19 vaccine booster for children aged 6 months through 4 years.
Pfizer applies for FDA authorization for Omicron-retooled vaccine booster in kids under 5Pfizer Inc and its German partner BioNTech SE said on Monday they have submitted an application to the U.S. Food and Drug Administration (FDA) for emergency use authorization of their Omicron-adapted COVID-19 vaccine booster for children aged 6 months through 4 years.
Read more »
