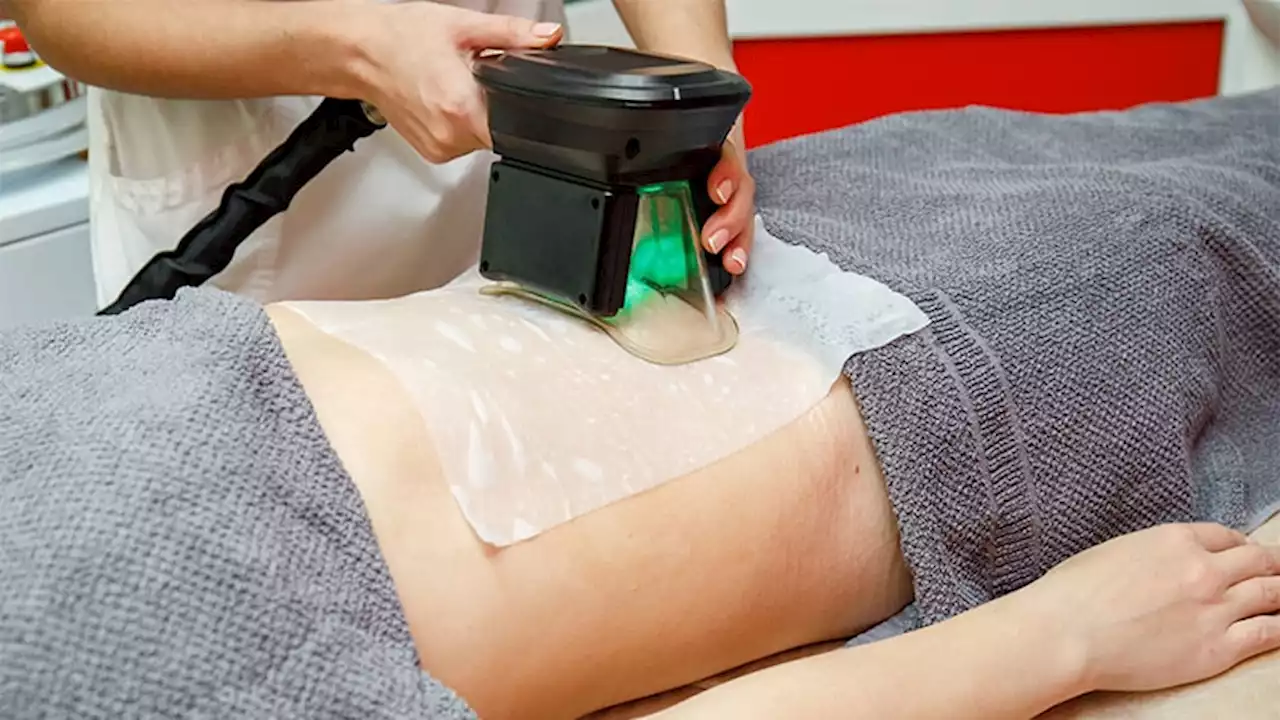
Cryolipolysis accounted for a majority of noninvasive cosmetic procedures associated with adverse events, according to an analysis of data.
August 26, 2022
The number of noninvasive body-contouring procedures performed in the United States increased by fivefold from 2011 to 2019, attributed in part to a combination of improved technology and new medical devices, as well as a"cosmetically savvy consumer base heavily influenced by social media," wrote Young Lim, MD, PhD, of the department of dermatology, Massachusetts General Hospital, Boston, and coauthors.
The researchers used the MAUDE database to identify and highlight adverse events associated with noninvasive body contouring technology in order to improve patient safety and satisfaction., they analyzed 723 medical device reports reported between 2015 and 2021: 660 for noninvasive body contouring, 55 for cellulite treatments, and 8 for muscle stimulation.
Overall, paradoxical hyperplasia accounted for the majority of adverse reactions in the noninvasive body-contouring category . In PAH, patients develop additional adipose tissue in areas treated with cryolipolysis. In this study, all reports of PAH as well as all 47 reported cases of abdominal hernias were attributed to the CoolSculpting device.
South Africa Latest News, South Africa Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA Approves Oral TYK2 Inhibitor for Treating PsoriasisDeucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, is the first drug in this class to be approved.
FDA Approves Oral TYK2 Inhibitor for Treating PsoriasisDeucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, is the first drug in this class to be approved.
Read more »
 FDA warns of rare cases of cancers possibly linked to breast implantsWhile rare, the FDA is warning about certain cancers, including squamous cell carcinoma and various lymphomas, that may develop in scar tissue that forms around breast implants.
FDA warns of rare cases of cancers possibly linked to breast implantsWhile rare, the FDA is warning about certain cancers, including squamous cell carcinoma and various lymphomas, that may develop in scar tissue that forms around breast implants.
Read more »
 Congress hits home stretch renewing FDA user fee programsThe FDA could begin sending furlough notices to staff if lawmakers don't settle on a mechanism for reviewing prescription drugs, medical devices, generic drugs and biosimilars.
Congress hits home stretch renewing FDA user fee programsThe FDA could begin sending furlough notices to staff if lawmakers don't settle on a mechanism for reviewing prescription drugs, medical devices, generic drugs and biosimilars.
Read more »
 More Than 200 Cases of a Bottled Starbucks Drink Have Been RecalledThey may have been contaminated with metal fragments, the FDA said.
More Than 200 Cases of a Bottled Starbucks Drink Have Been RecalledThey may have been contaminated with metal fragments, the FDA said.
Read more »
 Skin lightening products are a 'regulatory black hole,' and the FDA is warning against using themThe FDA's Skin Facts! Initiative is aimed at promoting safe use of skin care products, urging people to be aware of non-prescription products marketed to lighten or bleach the skin.
Skin lightening products are a 'regulatory black hole,' and the FDA is warning against using themThe FDA's Skin Facts! Initiative is aimed at promoting safe use of skin care products, urging people to be aware of non-prescription products marketed to lighten or bleach the skin.
Read more »
 FDA approves over-the-counter hearing aids to lower costs, increase accessEighty percent of people who would benefit from hearing aids don't wear them, according to the National Institutes of Health. That's because of cost, access and stigma. However, a new move by the FDA may change all of that.
FDA approves over-the-counter hearing aids to lower costs, increase accessEighty percent of people who would benefit from hearing aids don't wear them, according to the National Institutes of Health. That's because of cost, access and stigma. However, a new move by the FDA may change all of that.
Read more »