Doctors are urging the FDA to convene an expert panel to review safety concerns around an Alzheimer's drug that won accelerated approval in January, citing reports of 'brain swelling, brain bleeding and death.'
found that while the lecanemab trial represents "an important milestone," there's still a need for "a thorough appraisal of the data" to indicate whether the results are clinically meaningful.
Alzheimer's disease "is a very slowly progressive condition without treatment. And so, you add a treatment that slows it a little bit more," but "it's really not perceptible day-to-day to the patients and the families," said Constantine Lyketsos, an Alzheimer's researcher at Johns Hopkins School of Medicine.Lecanemab targets amyloid plaques, proteins in the brain that are believed to contribute to the development of Alzheimer's.
Lecanemab is the third drug that FDA has used beta amyloid PET scans "to decide whether they're going to grant accelerated approval, and yet we've never had a public discussion of the data to support the use of that endpoint," Karlawish added.
"Very positive results in the trial on multiple outcomes and biomarkers don't historically go to an advisory committee," she added. Apostolova told Axios that she has worked as a consultant for Eisai on lecanemab, and said that if an advisory meeting is called, she would not participate as a result.
South Africa Latest News, South Africa Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
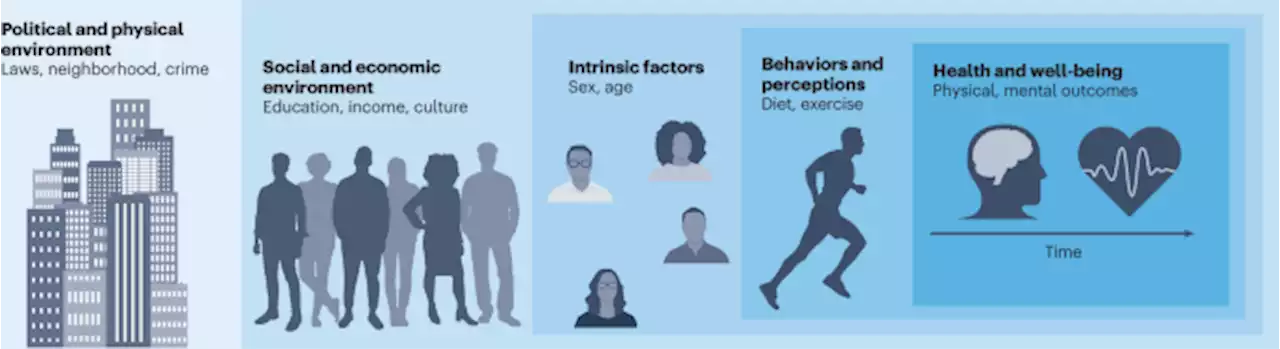 American Life in Realtime: a benchmark registry of health data for equitable precision health - Nature MedicineAmerican Life in Realtime: a benchmark registry of health data for equitable precision health. Correspondence from Ritika R. Chaturvedi UCSF and colleagues.
American Life in Realtime: a benchmark registry of health data for equitable precision health - Nature MedicineAmerican Life in Realtime: a benchmark registry of health data for equitable precision health. Correspondence from Ritika R. Chaturvedi UCSF and colleagues.
Read more »
 Similar Brain Atrophy in Obesity and Alzheimer's DiseaseGray matter thinning in patients with obesity correlates with that in patients with Alzheimer's disease. It mainly affects the right temporo-parietal cortex and left prefrontal cortex.
Similar Brain Atrophy in Obesity and Alzheimer's DiseaseGray matter thinning in patients with obesity correlates with that in patients with Alzheimer's disease. It mainly affects the right temporo-parietal cortex and left prefrontal cortex.
Read more »
 First drug proven to slow Alzheimer's won't be available to most patients for several monthsLeqembi, the first drug shown to slow Alzheimer’s, has been approved by the FDA, but most U.S. patients will not be able to receive treatment for months.
First drug proven to slow Alzheimer's won't be available to most patients for several monthsLeqembi, the first drug shown to slow Alzheimer’s, has been approved by the FDA, but most U.S. patients will not be able to receive treatment for months.
Read more »
 Elgin woman's book chronicles her unlikely path to motherhood through Alzheimer'sPam Singleton of Elgin has self-published a book titled 'My Mother, My Child: An Unusual Journey To Becoming A Mother Through Alzheimer's.' The book chronicles Singleton's time as a part-time caregiver to her afflicted mother, who came to think the author was her mother instead of her daughter.
Elgin woman's book chronicles her unlikely path to motherhood through Alzheimer'sPam Singleton of Elgin has self-published a book titled 'My Mother, My Child: An Unusual Journey To Becoming A Mother Through Alzheimer's.' The book chronicles Singleton's time as a part-time caregiver to her afflicted mother, who came to think the author was her mother instead of her daughter.
Read more »
 Why a new Alzheimer's drug is having a slow US debutTwo big factors behind the slow debut, experts say, are scant insurance coverage and a long setup time needed by many health systems.
Why a new Alzheimer's drug is having a slow US debutTwo big factors behind the slow debut, experts say, are scant insurance coverage and a long setup time needed by many health systems.
Read more »
