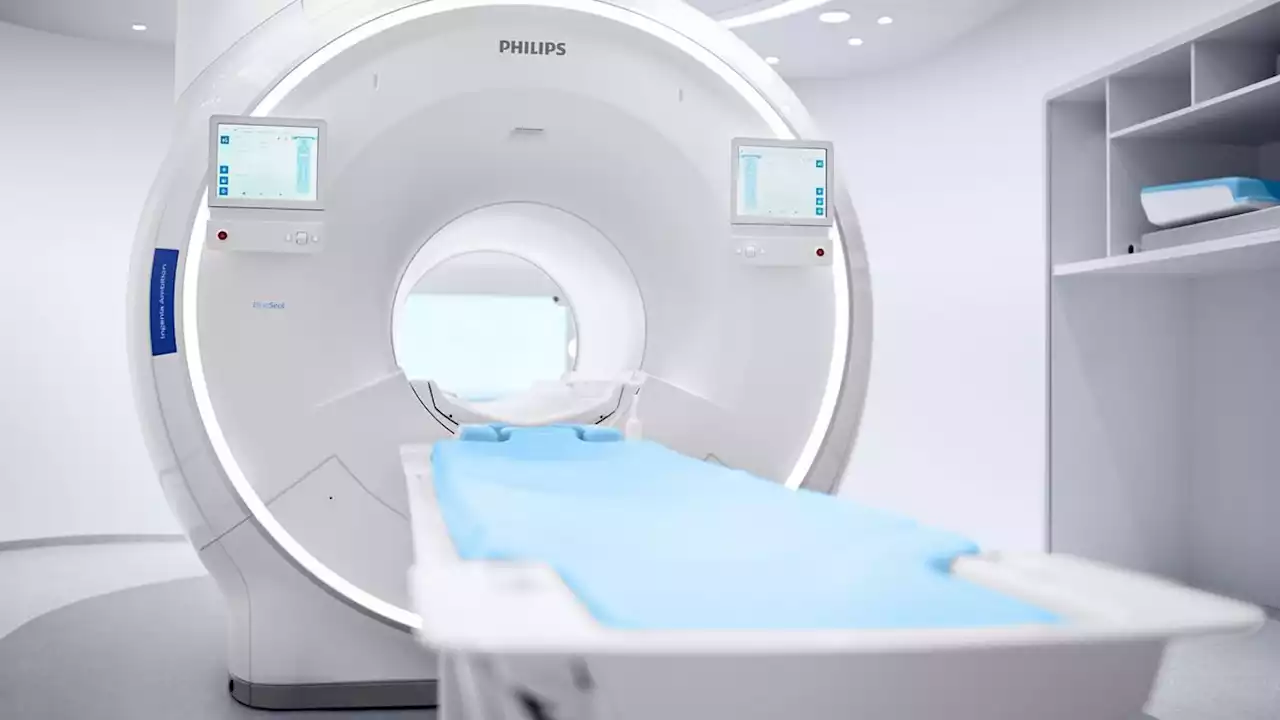
The artificial intelligence software speeds up the process of taking scans.
, requires device manufacturers to register, and notify FDA of their intent to market a medical device at least 90 days in advance.MRCAT Head and Neck, short for MR for Calculating Attenuation, allows for the use of MRI as the main imagining modality without oncologists needing to use a CT scan. The AI software creates images that look like CT scan images, in terms of accuracy and resolution. The MRCAT takes less than three minutes to complete.
. It was said to be the industry-first online marketplace that offered curated, readily available AI resources.“The quality of your AI is only as good as the quality of the data you feed into it,” said Jeroen Tas, chief innovation & strategy officer at Philips. “We have designed HealthSuite Insights to be used by the people who work with patient data on a daily basis and have the contextual understanding, including doctors, clinicians and hospital managers.
South Africa Latest News, South Africa Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
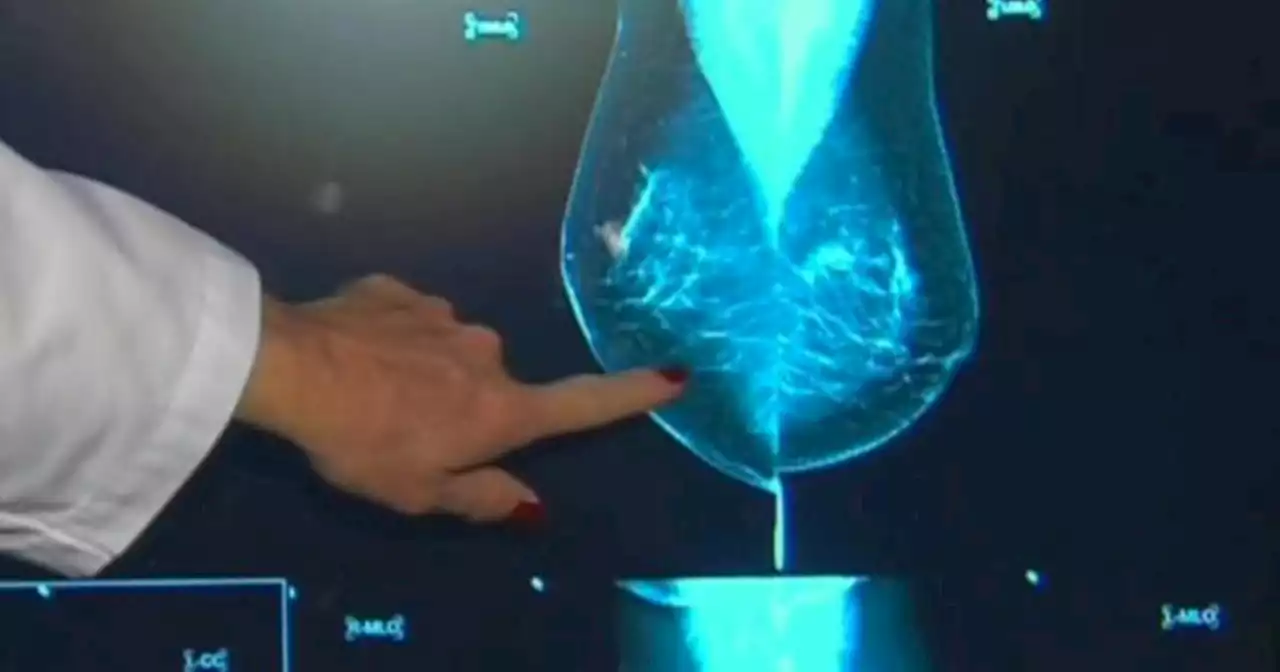 FDA plans new regulations on mammograms in effort to help women with dense breastsMammograms can sometimes fail to detect tumors in women who have a higher breast density. As a result, the FDA said it's planning new regulations for informing women about their tissue type and screening options.
FDA plans new regulations on mammograms in effort to help women with dense breastsMammograms can sometimes fail to detect tumors in women who have a higher breast density. As a result, the FDA said it's planning new regulations for informing women about their tissue type and screening options.
Read more »
 FDA Approves New Immunotherapy Combo for Liver Cancer➡️ FDAapproves a new immunotherapy combination for use in the treatment of unresectable HCC, the most common type of liver cancer. Single dose of Tremelimumab then Regular Interval Durvalumab. STRIDE MedTwitter
FDA Approves New Immunotherapy Combo for Liver Cancer➡️ FDAapproves a new immunotherapy combination for use in the treatment of unresectable HCC, the most common type of liver cancer. Single dose of Tremelimumab then Regular Interval Durvalumab. STRIDE MedTwitter
Read more »
 FDA Approves Upadacitinib (Rinvoq) for Sixth Indication✅ FDAapproves a sixth indication for JAK inhibitor upadacitinib -- for adults with nonradiographic axial spondyloarthritis meeting certain criteria. RheumTwitter
FDA Approves Upadacitinib (Rinvoq) for Sixth Indication✅ FDAapproves a sixth indication for JAK inhibitor upadacitinib -- for adults with nonradiographic axial spondyloarthritis meeting certain criteria. RheumTwitter
Read more »
 FDA urges Mighty Bliss electric heating pad customers to stop using them citing injury riskThe recall covers hundreds of thousands of the heating pads distributed and sold, with customers urged to stop using them because of the risk of burns and other issues.
FDA urges Mighty Bliss electric heating pad customers to stop using them citing injury riskThe recall covers hundreds of thousands of the heating pads distributed and sold, with customers urged to stop using them because of the risk of burns and other issues.
Read more »
 Horrifying – New Study Indicates That Popular Sugar Substitutes Worsen Your MemoryUsing laboratory models, scientists discovered that ingesting FDA-approved levels of saccharin, ACE-K, and stevia early in life may result in many changes to the body, including brain areas linked to memory and reward-motivated behavior. Early-life high-sugar diets have been linked to impaired br
Horrifying – New Study Indicates That Popular Sugar Substitutes Worsen Your MemoryUsing laboratory models, scientists discovered that ingesting FDA-approved levels of saccharin, ACE-K, and stevia early in life may result in many changes to the body, including brain areas linked to memory and reward-motivated behavior. Early-life high-sugar diets have been linked to impaired br
Read more »