FDA authorizes a coronavirus booster shot for children as young as 5 as cases rise nationally
Tuesday’s move may sharpen some parents’ frustration about the divide between children who are able to get a third shot and those who can’t yet get a dose. Children younger than 5 still lack access to any vaccine. A top FDA officialthat the first shots might become available in June, assuming data supports such a move.
The FDA has set aside several dates — June 8, 21 and 22 — to meet with its outside vaccine advisers — the Vaccines and Related Biological Products Advisory Committee — to discuss applications for pediatric vaccines.The FDA also announced plans to meet June 7 with its advisers to talk about an emergency authorization request for a coronavirus vaccine made by Novavax for people 18 and older.
South Africa Latest News, South Africa Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
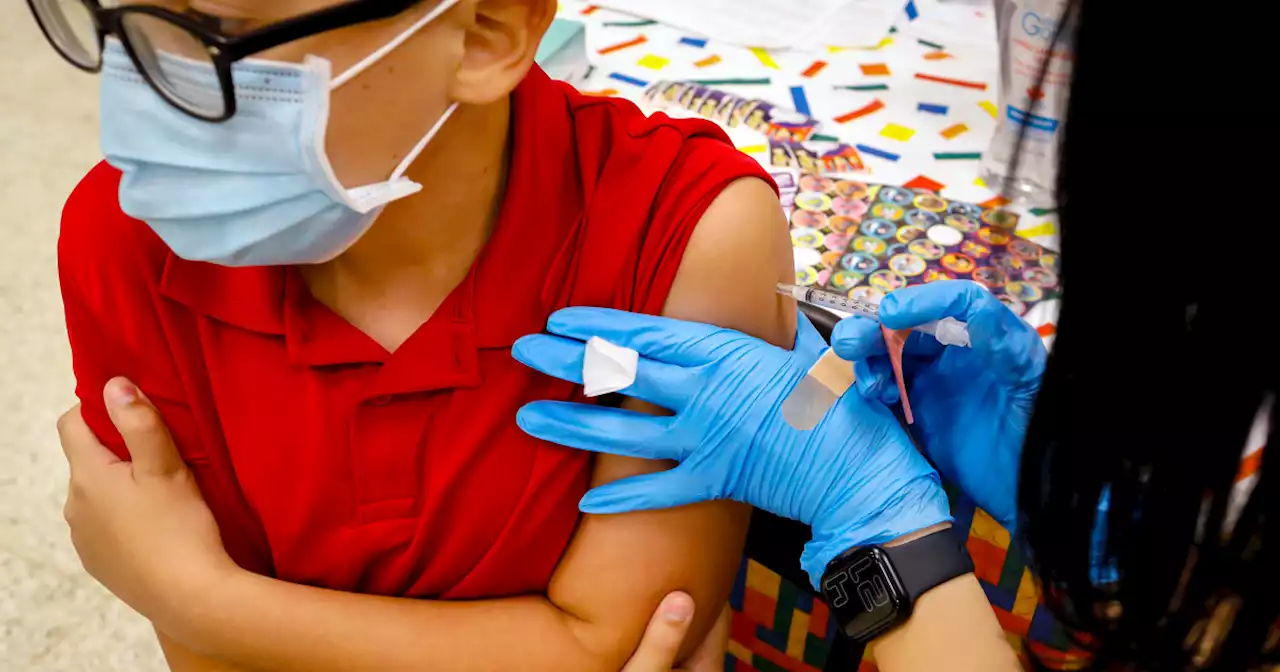 FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
Read more »
 FDA Authorizes Pfizer Covid Booster Dose for Kids Ages 5 to 11 Years OldThe Food and Drug Administration on Tuesday authorized a Pfizer booster dose for children ages 5 through 11 years old at least five months after they complete their two-dose primary series. Dr. Peter Marks, head of the FDA division responsible for vaccines, said data increasingly shows that the protection provided by two shots wanes off over time. The FDA determined…
FDA Authorizes Pfizer Covid Booster Dose for Kids Ages 5 to 11 Years OldThe Food and Drug Administration on Tuesday authorized a Pfizer booster dose for children ages 5 through 11 years old at least five months after they complete their two-dose primary series. Dr. Peter Marks, head of the FDA division responsible for vaccines, said data increasingly shows that the protection provided by two shots wanes off over time. The FDA determined…
Read more »
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Read more »
 FDA Authorizes Pfizer Booster for Kids 5-11, Sends to CDC for ApprovalBoosters could become available as early as Friday if approved by the CDC, which could come Thursday.
FDA Authorizes Pfizer Booster for Kids 5-11, Sends to CDC for ApprovalBoosters could become available as early as Friday if approved by the CDC, which could come Thursday.
Read more »
 FDA approves Pfizer COVID-19 booster for children ages 5 to 11Pfizer’s COVID-19 booster shot for U.S. kids ages 5 to 11 has been approved by the FDA. Next, it will need the CDC’s final sign-off.
FDA approves Pfizer COVID-19 booster for children ages 5 to 11Pfizer’s COVID-19 booster shot for U.S. kids ages 5 to 11 has been approved by the FDA. Next, it will need the CDC’s final sign-off.
Read more »
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Read more »
