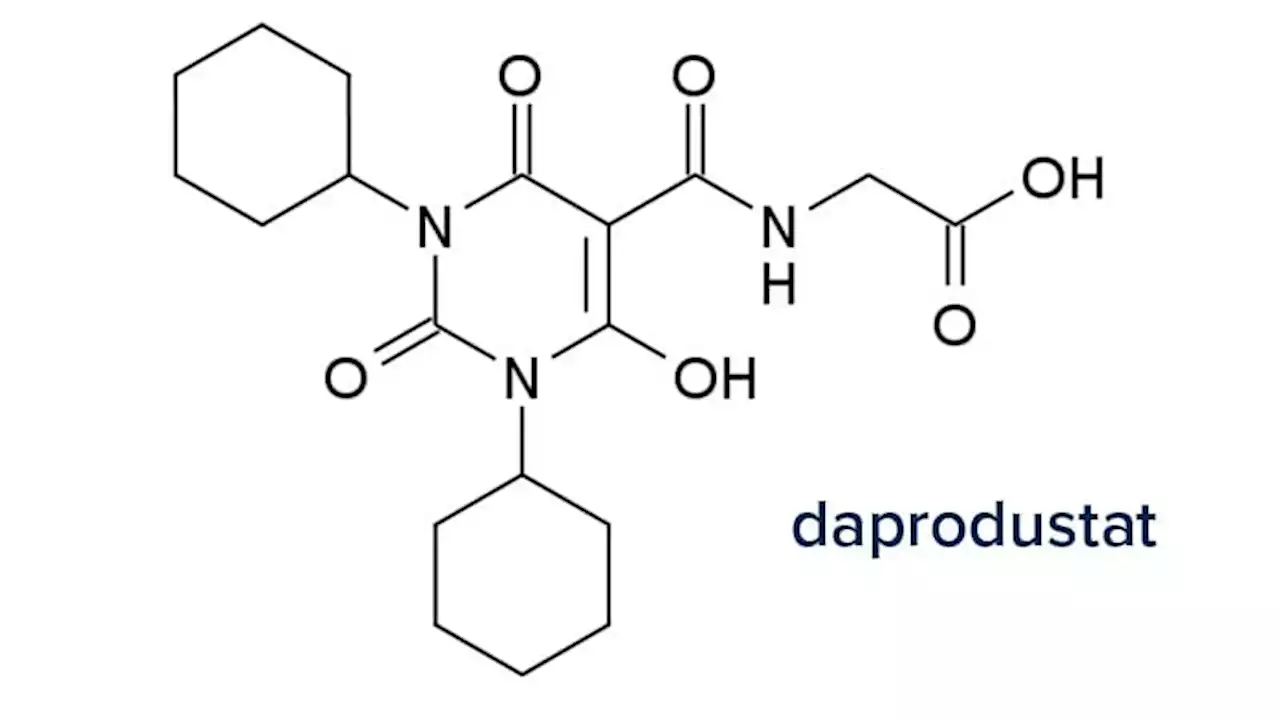
FDA approves daprodustat, first oral anemia treatment
The FDA's approval statement also listed the most common adverse effects of daprodustat as hypertension, thrombotic vascular events, abdominal pain, dizziness, and allergic reactions. In addition, the agency advised that patients should not use daprodustat if they also take certain drugs that cause increased serum levels of the drug or if they have uncontrolled high blood pressure.
The drug class that daprodustat, roxadustat, and vadadustat all belong to is the hypoxia-inducible factor prolyl hydroxylase inhibitors, which stabilize HIF and thereby increase the secretion of endogenousand the production of red cells. This approach to resolving anemia mimics the physiologic effects that occur in people when they are at high altitudes.
According to the GSK statement, CKD affects approximately 39 million people in the United States and about 6 million of these people also have anemia. Approximately 810,000 of these people with CKD have end-stage renal disease, including about 558,000 patients on treatment with dialysis. The ASCEND-D and ASCEND-ND trials were sponsored by GSK. Farrell and Penzenstadler had no disclosures.