The Food and Drug Administration on Wednesday authorized Pfizer’s and Moderna’s updated COVID-19 booster shots that target the BA.4 and BA.5 omicron variants.
CDC panel clears updated Pfizer, Moderna COVID boosters targeting omicron subvariantsPUBLISHED 10:00 AM ET Aug. 31, 2022authorized Pfizer’s and Moderna’s updated COVID-19 booster shotsOn Thursday, an advisory panel for the Centers for Disease Control and Prevention also recommended the vaccines, pushing them one step closer to being offered to Americans across the country.
The CDC’s Advisory Committee on Immunization Practices, which votes on whether the agency should issue recommendations, held meetings Thursday to discuss approving the vaccines. CDC Director Dr. Rochelle Walensky will make the final call on the shots, which could come within hours of a vote by the advisers. Shots could then begin going into arms within a day.
Sánchez was ultimately the only opposing vote, saying he felt the decision “was a bit premature,” though he acknowledged the vaccines were likely to be found safe through rigorous human testing. But other panel members agreed that waiting to approve the updated vaccine until clinical data is available, which will not be until November, would prove more harmful in the long run.
Pfizer’s booster, which is manufactured in partnership with the German company BioNTech, would be available to people 12 and older, while individuals 18 and older would be eligible for Moderna’s. The FDA said it will work quickly to evaluate future data and submissions for bivalent boosters for other age groups.
“The FDA considers such data as relevant and supportive of vaccines containing a component of the omicron variant BA.4 and BA.5 lineages,” the agency’s announcement said. “The public can be assured that a great deal of care has been taken by the FDA to ensure that these bivalent COVID-19 vaccines meet our rigorous safety, effectiveness and manufacturing quality standards for emergency use authorization,” Marks added.
South Africa Latest News, South Africa Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
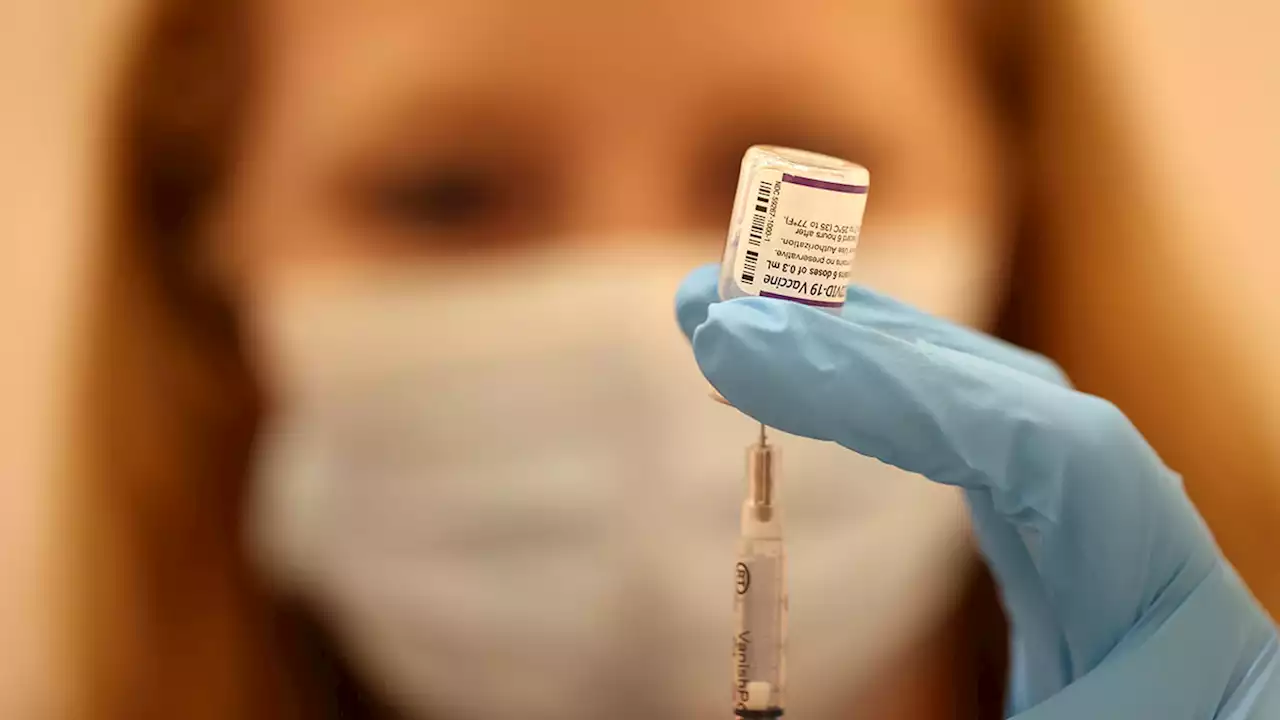 CDC Advisers Weigh Who Needs Updated COVID Booster and WhenGovernment advisers are debating who should get updated COVID-19 boosters and when. The tweaked shots made by Pfizer and rival Moderna promise Americans a chance at their most up-to-date protection ahead of an expected winter surge because they target the omicron strains now circulating. But it’s not clear just how much more protection they’ll get. Another key question: How long people should wait after their last shot to get the most benefit from a new one. The Centers for Disease Control and Prevention will issue recommendations soon, the last step before updated boosters can roll out.
CDC Advisers Weigh Who Needs Updated COVID Booster and WhenGovernment advisers are debating who should get updated COVID-19 boosters and when. The tweaked shots made by Pfizer and rival Moderna promise Americans a chance at their most up-to-date protection ahead of an expected winter surge because they target the omicron strains now circulating. But it’s not clear just how much more protection they’ll get. Another key question: How long people should wait after their last shot to get the most benefit from a new one. The Centers for Disease Control and Prevention will issue recommendations soon, the last step before updated boosters can roll out.
Read more »
 Covid-19 Boosters Targeting Omicron Reviewed by CDC AdvisersThe latest booster-shot campaign, which health authorities have been counting on to protect people once the weather turns colder and cases often rise, could start after Labor Day.
Covid-19 Boosters Targeting Omicron Reviewed by CDC AdvisersThe latest booster-shot campaign, which health authorities have been counting on to protect people once the weather turns colder and cases often rise, could start after Labor Day.
Read more »
 Infant Mortality | Maternal and Infant Health | Reproductive Health | CDCIn 2020, the infant mortality rate in the United States was 5.4 deaths per 1,000 live births. CDC is committed to improving birth outcomes and reduce infant mortality in the United States.
Infant Mortality | Maternal and Infant Health | Reproductive Health | CDCIn 2020, the infant mortality rate in the United States was 5.4 deaths per 1,000 live births. CDC is committed to improving birth outcomes and reduce infant mortality in the United States.
Read more »
 CDC advisers weigh who needs updated COVID booster and whenGovernment advisers are debating who should should get new updated COVID-19 boosters and when
CDC advisers weigh who needs updated COVID booster and whenGovernment advisers are debating who should should get new updated COVID-19 boosters and when
Read more »
 CDC recommends new booster shots to fight omicronAn advisory committee to the CDC has endorsed updated versions of the Moderna and Pfizer-BioNTech vaccines that target the original coronavirus and two omicron subvariants.
CDC recommends new booster shots to fight omicronAn advisory committee to the CDC has endorsed updated versions of the Moderna and Pfizer-BioNTech vaccines that target the original coronavirus and two omicron subvariants.
Read more »
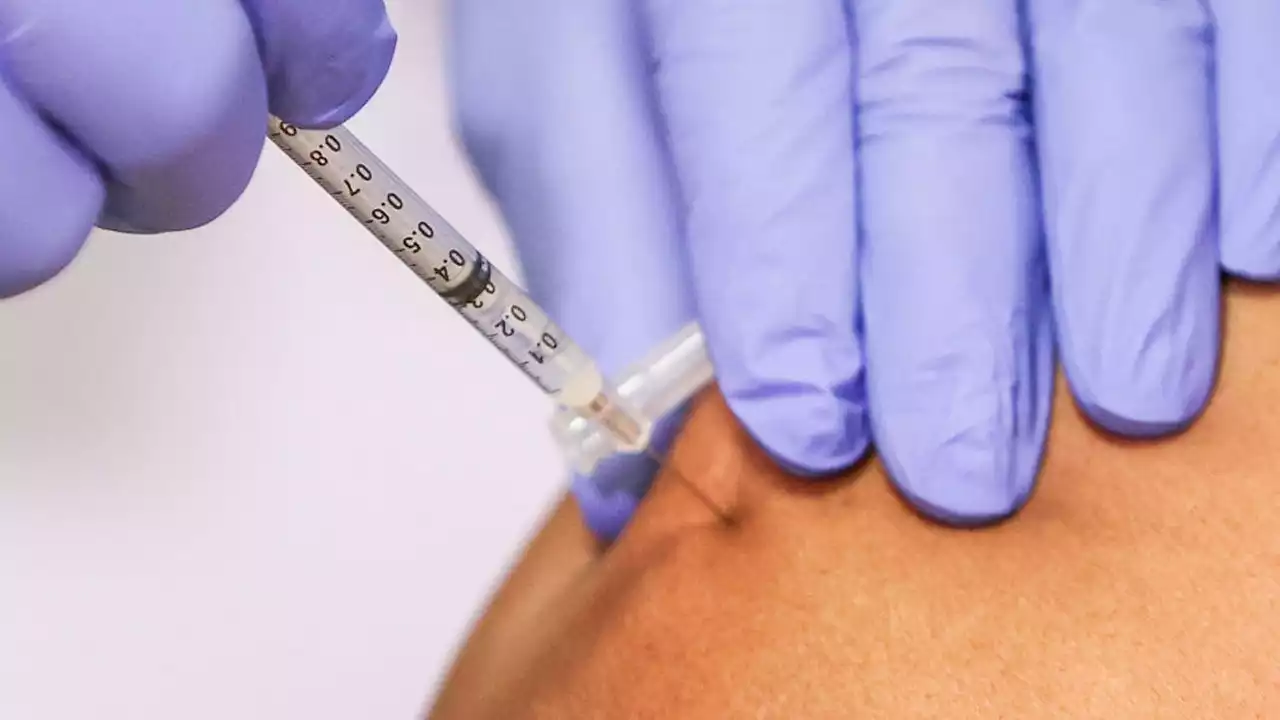 CDC panel recommends tweaked COVID-19 boosterThe tweaked COVID booster, which was approved in a fast-tracked strategy, is expected to be available next week after Labor Day.
CDC panel recommends tweaked COVID-19 boosterThe tweaked COVID booster, which was approved in a fast-tracked strategy, is expected to be available next week after Labor Day.
Read more »
